SURCRYL Absorbable Sutures – Trusted Polyglycolic for Safe & Reliable Healing
Absorbable Polyglycolic Acid Synthetic Braided Surgical Sutures
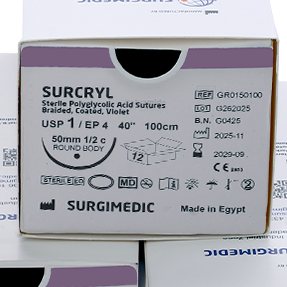
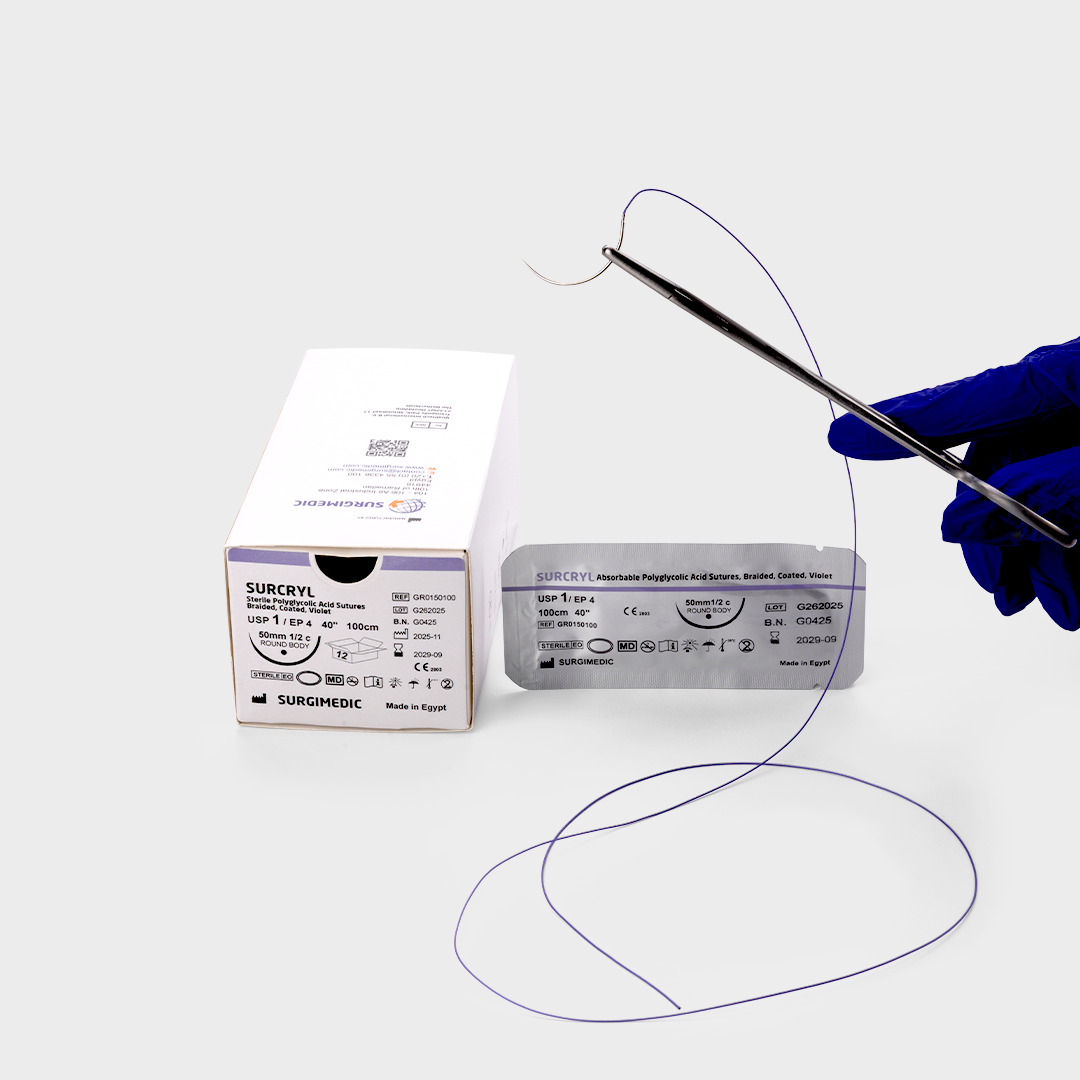
SURCRYL absorbable sutures is a violet, braided, and coated multifilament suture made of polyglycolic acid with polycaprolactone and calcium stearate coating. It offers high tensile strength, excellent handling, knot security, and smooth tissue passage. Absorbed in 60-90 days, it’s ideal for general soft tissue closure in various surgeries except cardiovascular and neurosurgery.
| Type | First and foremost, SURCRYL is a braided and coated multifilament absorbable suture, specifically engineered for superior handling, secure knotting, and smooth tissue passage. Moreover, as a premium absorbable surgical suture, it offers dependable performance in both general and specialized surgeries. |
| Composition | From a material standpoint, SURCRYL is manufactured from Polyglycolic Acid. Accordingly, it delivers predictable hydrolytic absorption with optimal tensile strength. As a result, this advanced formulation makes SURCRYL one of the most reliable Polyglycolic acid absorbable sutures available today. |
| Color | For enhanced intraoperative visibility, SURCRYL is available in violet color, which therefore improves precision during surgical procedures. |
| Coating | In addition, each suture is coated with a precise layer of Polycaprolactone and Calcium Stearate (1%), ensuring: Reduced tissue drag, and thus smoother passage Enhanced knot security, for greater surgical confidence Excellent handling during surgery, even in delicate procedures Consequently, this coating significantly improves performance compared to uncoated absorbable sutures. |
| Tissue Reaction | Importantly, SURCRYL causes minimal tissue reaction, thereby promoting safe, gentle, and comfortable healing. |
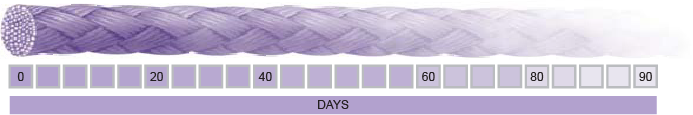
Absorption Profile of SURCRYL Polyglycolic acid Absorbable Sutures
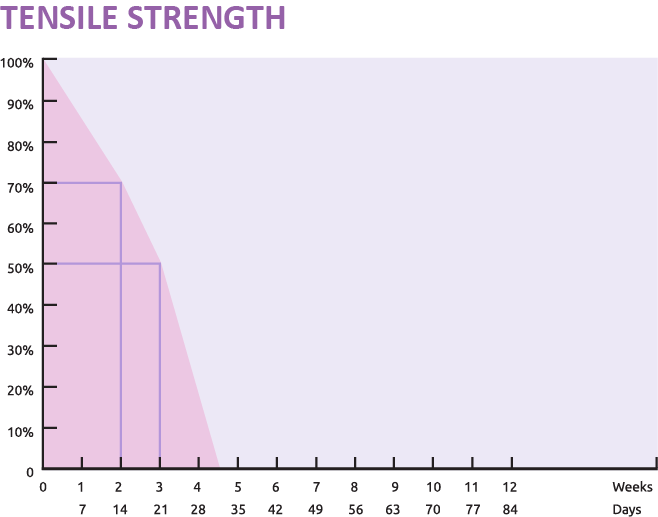
With regard to absorption, SURCRYL absorbable sutures are absorbed by hydrolytic action, with:
- total absorption in approximately 60 to 90 days.
- About 70% tensile strength retention after 2 weeks
- Around 50% tensile strength retention after 3 weeks
- Therefore, this predictable absorption profile makes SURCRYL ideal for soft tissue approximation that requires temporary yet reliable wound support.
Indications for Use – Where SURCRYL Absorbable Sutures Are Used
Based on its proven properties, SURCRYL is recommended for general soft tissue closure and ligation, including:
- General surgery.
- Skin closure.
- Gastrointestinal surgery.
- Plastic surgery.
- Gynaecology.
- Urology.
- Ophthalmic surgery.
- Orthopaedics.
As such, SURCRYL offers versatile clinical applications across multiple specialties.
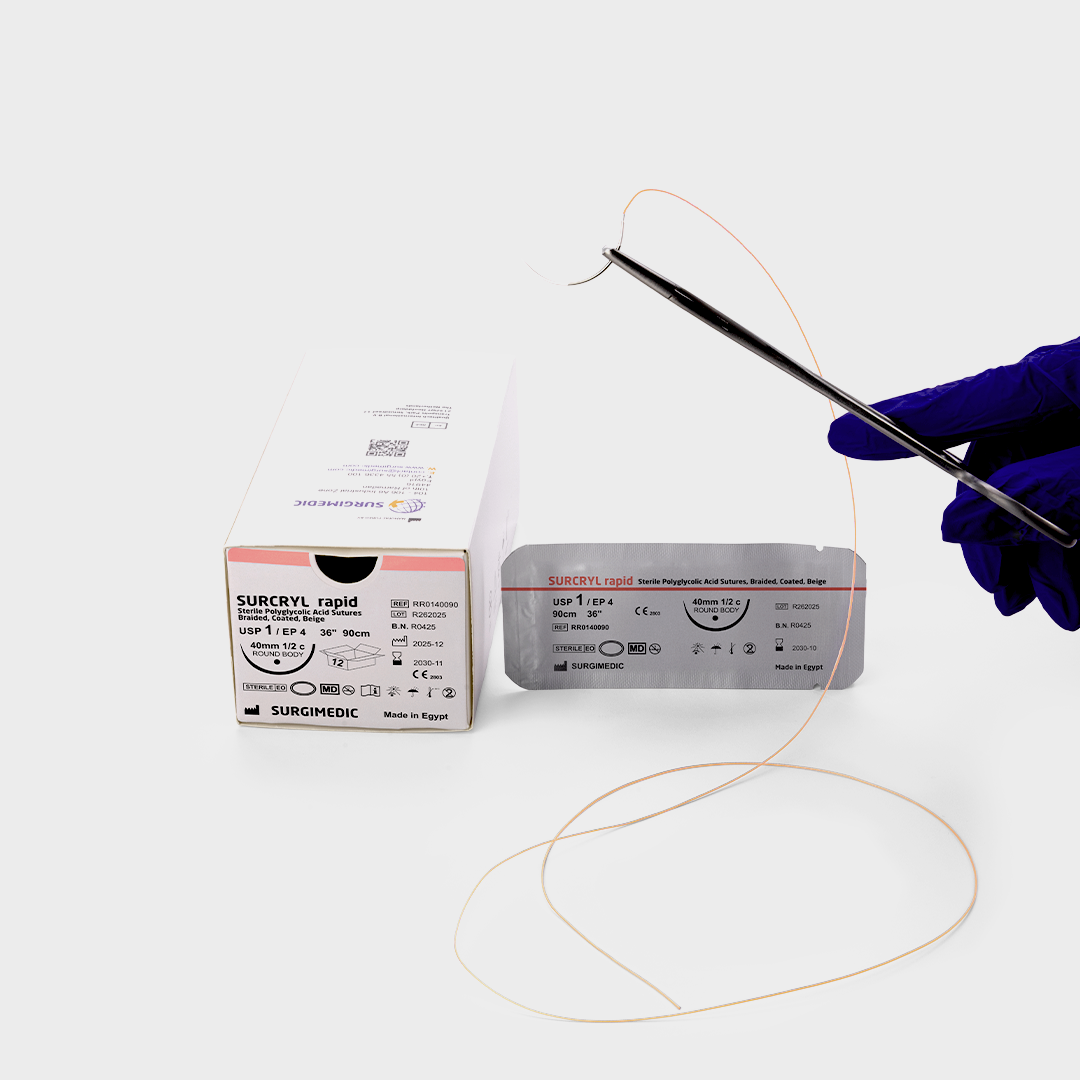
Indications for Use – Where SURCRYL™ RAPID Absorbable Sutures Are Used
Based on its proven properties, SURCRYL RAPID is recommended for general soft tissue closure and ligation, including:
- Plastic surgery
- Obstetrics (Episiotomy repair)
- Gynaecology
- Urology (Circumcision)
- Ophthalmic surgery (Conjunctiva)
- Pediatric surgery
As such, SURCRYL RAPID offers versatile clinical applications across multiple specialties.
Contradictions
However, SURCRYL absorbable sutures should not be used in:
- Cardiovascular surgery
- Neurosurgery
- Procedures requiring extended or permanent wound support
For this reason, proper case selection is essential for optimal outcomes.
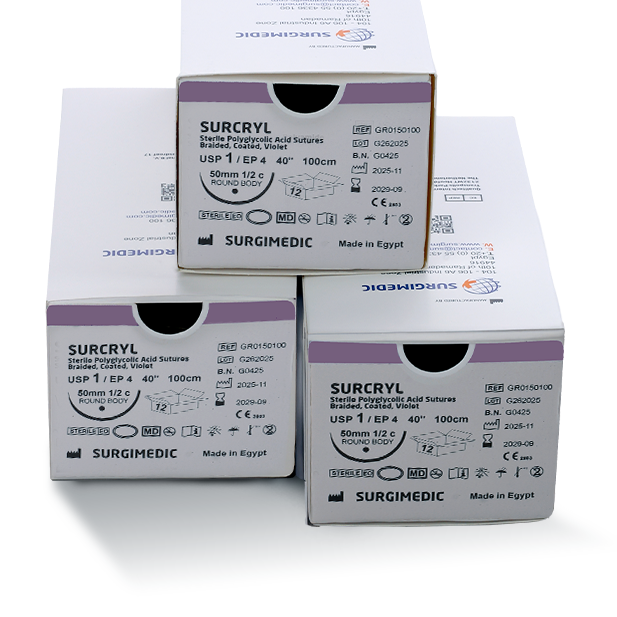
Sizes & Packaging – Direct from a Surgical Sutures Manufacturer
As a professional surgical sutures manufacturer, SURCRYL is supplied in:
- USP sizes 6/0 through 2 (metric 0.7 – 5.0)
- One dozen sutures per box with needles
- Three-dozen boxes available upon customer request
- Furthermore, bulk supply, private labeling, and OEM manufacturing are available for distributors and hospitals worldwide.
Sizes & Packaging – Direct from a Surgical Sutures Manufacturer
As a professional surgical sutures manufacturer, SURCRYL RAPID is supplied in:
- USP sizes 6/0 through 1 (metric 0.7 – 4.0)
- One dozen sutures per box with needles
- Three-dozen boxes available upon customer request
Furthermore, bulk supply, private labeling, and OEM manufacturing are available for distributors and hospitals worldwide.

 For reference purposes see the provided
For reference purposes see the provided 